The study indicates that the layer of fat that involves blood vessels may be responsible for aggravating the disease
By Guilherme Gama
Heart failure is a syndrome characterized by the incapacity of the heart to maintain an appropriate blood flow to the various organs and tissues of the body. According to data from the Ministry of Health, in the last ten years, heart failure was responsible for 228,239 deaths, with 3,047 being in the first two months of this year alone. Currently, there is a concern with the increase in such numbers as a function of the COVID-19 cases, which is related to the dysfunction of this organ.

With the objective of understanding this disease that compromises public health, researchers from the Laboratory of Vascular Physiology of the USP Institute of Biomedical Sciences (ICB) began investigating beyond the heart and sought to understand the behavior of the main blood vessels near the organ. The new study demonstrated that the layer of fat that involves such vessels, called the perivascular adipose tissue, is related to the loss of the capacity of the vessels to adapt to the pumped blood flow. This incapacity stems from a lack in the anticontractile function of this tissue, which overloads the functioning of the heart and may aggravate the disease. The unpublished data open new perspectives for studies in the field and point to the perivascular adipose tissue as a new target for medical treatments aiming to improve the clinical picture of this complex syndrome.
The study is part of the doctoral research by Milene Tavares Fontes, with the supervision of the laboratory coordinator, professor Luciana Venturini Rossoni, and the support of the São Paulo Research Foundation (FAPESP). The article entitled Renin–angiotensin system overactivation in perivascular adipose tissue contributes to vascular dysfunction in heart failure was published in the Clinical Science journal in December 2020.
“We were the first group, internationally, to show that perivascular fat lost the physiological function in heart failure, which is now a disease not only of the heart and the endothelial layer, the thin internal layer of blood vessels, but also of perivascular fat”, states the supervisor of the work, Luciana Rossoni.
In heart failure, the large vessels that exit the heart in the direction of the other organs and tissues, the arteries (such as the aorta), present a dysfunction: an exacerbated correction that stiffens them and hampers their malleability, a characteristic that allows the ideal blood flow. One of the systems that regulate this contraction of the vessels is denominated “renin-angiotensin”, responsible for the release of hormones that, through the action of receptors, can contract and/or relax the vessels, such as angiotensin II. Studies so far forgotten in the scientific literature indicated that the perivascular adipose tissue produced substances that controlled vascular contraction, as well as constituents of this system. The ICB scientists recovered these data and sought to verify whether or not this layer of fat kept exercising this physiological function in the arteries in heart failure.
Why is this anticontractile effect so important?
According to Luciana, the first vessel that receives the volume that the left ventricle (heart chamber) ejects to be distributed throughout the organism is the aorta artery. She exemplifies that if this vessel loses the capacity to deform and accommodate the ejected blood volume, a resistance against the pump function of the heart is generated. This dysfunction is concerning when it represents a resistance to the heart that is not functioning normally. “This is what we call overload, that worsens the pumping of the heart and generates a vicious circle for the worsening of the heart failure”, she stated to the Jornal da USP.
To assess the role of the external fat that coats this artery, model animals were induced to heart failure by the infarction of the myocardium, the heart tissue, through the ligature of the coronary artery, upon interrupting the blood flow that nourishes a portion of the heart tissue. Twelve weeks after the surgery and the confirmation of the heart failure, the aortas of the animals were analyzed through a range of hemodynamic, biochemical, molecular, and functional tests in vivo and in vitro to measure the activity of the enzymes of the renin-angiotensin system, the expression of the constituents of this system, and also the contraction response of the vessels.
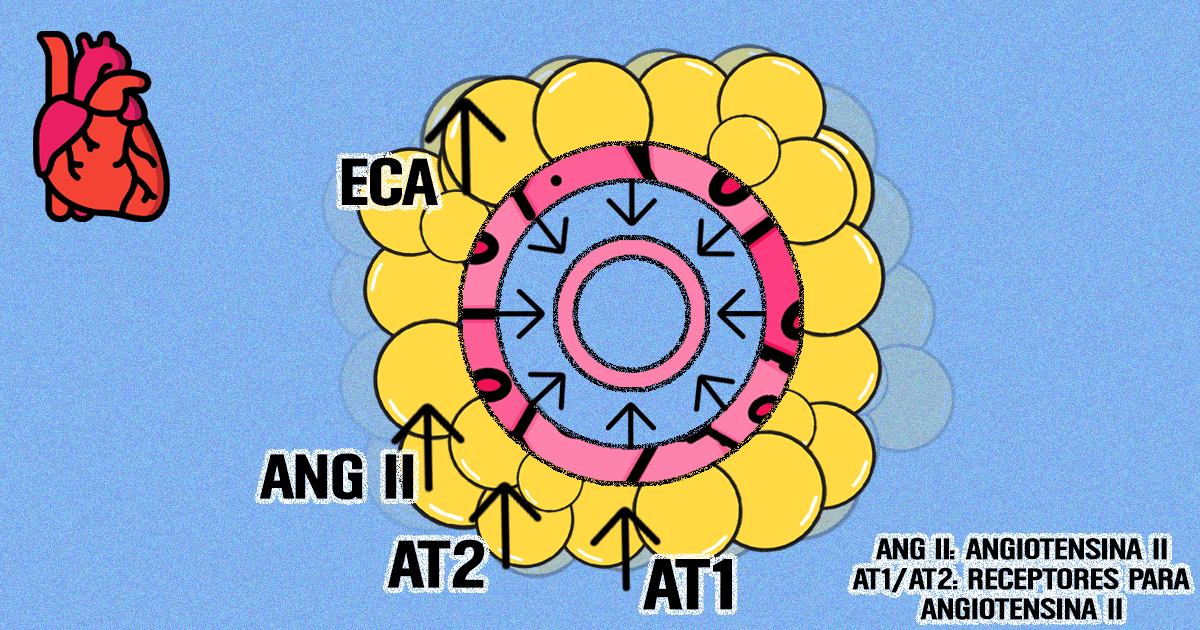
The results showed that, compared to the healthy animals, the perivascular fat of the animals with heart failure presented an increase in the activity of the angiotensin-converting enzyme, a key enzyme of this system for the production of angiotensin II, and of angiotensin II itself. In turn, the vessels of these animals presented a loss of the anticontractile effect of the perivascular fat, which was reversed with the acute blocking of the angiotensin II receptors.
The study showed that the loss of function of the layer of fat that coats the vessels and the activation of the renin-angiotensin system in this tissue have a primordial role in activating the contraction system in the vessels, being a determining factor for the evolution or worsening of the disease.

“We were the first group, internationally, to show that perivascular fat lost the physiological function in heart failure, which is now a disease not only of the heart and the endothelial layer, the thin internal layer of blood vessels, but also of perivascular fat”, states Luciana.
The scientist also explains that the perivascular fat in the aorta is predominantly formed by brown, healthy fat and that the data also pointed out that the fat that coated the aorta of the animals in heart failure changed from brown to white, from a good characteristic to a bad one.
“The study showed that the perivascular adipose tissue might be an important target for new therapies”, said the researcher to the Jornal da USP. She stresses that the data set a precedent for the development of new maneuvers that may improve the condition of this fat and, thus, improve the quality of the vessel and the overloading of the heart.
The next step of the research is aimed toward the application of physical exercises in sick animals as a possible strategy for improving the condition of heart failure. The doctoral student advances that the data are promising: “The results are demonstrating that physical training is capable of reversing part of the damages caused by heart failure in this layer of fat. Physical exercise may be used as a non-pharmacological tool, a coadjuvant in the treatment of heart failure”, states Milene.